
We
do the highest resolution etching in the world!
1,200 dpi images @
133 lpi
Titanium
Titanium
is an element (symbol Ti) like gold, silver and platinum.
Its atomic number is 22, with atomic weight of 47.9. It is
a silvery white non ferrous metal with the highest strength
to weight ratio of any known element. For this reason titanium
is the favored material in the aerospace industry. 85% of
the structural components in the Space Shuttle are made of
titanium. Titanium has been embraced by the medical world
for its superior bio-compatibility and is the preferred material
for surgical instruments. Titanium is inert and therefore
completely corrosion resistant. It does not react to salt
water, sunlight, microbes, or body/common chemistry.
 A
stable, substantially inert oxide film provides the material
with outstanding resistance to corrosion in a wide range
of aggressive media. Whenever fresh titanium is exposed to
the atmosphere or to any environment containing oxygen, it
immediately acquires a thin tenacious film of oxide. It is
the presence of this surface film that confers on the material
its excellent corrosion resistance. Provided that sufficient
oxygen is present, the film is self
healing and re-forms almost at once
if mechanically damaged.
A
stable, substantially inert oxide film provides the material
with outstanding resistance to corrosion in a wide range
of aggressive media. Whenever fresh titanium is exposed to
the atmosphere or to any environment containing oxygen, it
immediately acquires a thin tenacious film of oxide. It is
the presence of this surface film that confers on the material
its excellent corrosion resistance. Provided that sufficient
oxygen is present, the film is self
healing and re-forms almost at once
if mechanically damaged.
This
is the premier metal for our tributes. We have developed
a technique to image it, but it is expensive. The piece pictured
above is a photo of a small titanium tribute. And just to
show people that the metal is actually etched we removed
the surface coating on a test sample, the train piece pictured
below. You can still see the image quite well even though
there is no surface color providing contrast. Dark areas
are shadows caused by light refraction on the smooth vs.
etched surface.
Oxidation
 There
is good oxidation and bad oxidation. Oxidation is the interaction
between oxygen molecules and the different substances they
may contact. Not all oxygen interaction destroys metal. In
the case of iron it causes a slow burn which manifests itself
as rust. That eventually destroys the metal. In the case
of copper, oxidation forms a protective, greenish to black
patina of copper oxide compounds. When bronze oxidizes it
get darker. Some people like it, others do not. The metal
is not harmed. Many manufacturers coat the bronze with a
lacquer or other organic coating to retard oxidation. You
must keep re-coating the bronze with a barrier film or wax
it - like they do bronze sculptures. Manufacturers will apply
several coats of a barrier (paint-usually dark brown) to
a bronze headstone plaque. Once the barrier film is dry,
they will rub areas of the bronze plaque using a solvent
to remove areas of paint to expose the top edge of the letters
and sculpted design features to reveal the natural bronze
coloring, which in turn must be coated with a clear barrier
film. This provides a beautiful contrast to the dark brown
background. One potential problem with photo etched bronze
is the oxidation. When the piece oxidizes and starts to get
darker the contrast produced by the rubbed patina will be
lost and so will the image’s appearance.
There
is good oxidation and bad oxidation. Oxidation is the interaction
between oxygen molecules and the different substances they
may contact. Not all oxygen interaction destroys metal. In
the case of iron it causes a slow burn which manifests itself
as rust. That eventually destroys the metal. In the case
of copper, oxidation forms a protective, greenish to black
patina of copper oxide compounds. When bronze oxidizes it
get darker. Some people like it, others do not. The metal
is not harmed. Many manufacturers coat the bronze with a
lacquer or other organic coating to retard oxidation. You
must keep re-coating the bronze with a barrier film or wax
it - like they do bronze sculptures. Manufacturers will apply
several coats of a barrier (paint-usually dark brown) to
a bronze headstone plaque. Once the barrier film is dry,
they will rub areas of the bronze plaque using a solvent
to remove areas of paint to expose the top edge of the letters
and sculpted design features to reveal the natural bronze
coloring, which in turn must be coated with a clear barrier
film. This provides a beautiful contrast to the dark brown
background. One potential problem with photo etched bronze
is the oxidation. When the piece oxidizes and starts to get
darker the contrast produced by the rubbed patina will be
lost and so will the image’s appearance.
PASSIVE
FILM: The major characteristic of stainless is its ability
to form a thin layer of protection called a "passive
film" on its outside surface. This film results from
a continual process of low-level oxidation, so oxygen from
the atmosphere is needed for the passive film to exist. Once
formed, it prevents further oxidation or corrosion from occurring.
Even if chipped or scratched, a new passive film on stainless
will form. It’s “self healing” like titanium.
That’s why we use 316L stainless and titanium.
316
L Stainless Steel
Surgical Stainless
Surgical
stainless steel is a variation of steel consisting of an
alloy of chromium (12-20%), molybdenum (0.2-3%), and sometimes
nickel (8-12%). The chromium gives the metal its sheen, scratch-resistance
and corrosion resistance. The molybdenum gives corrosion-resistance,
and helps maintaining a cutting edge.
Although
there are myriads of variations in the recipes, there are
two main varieties of stainless steel; martensitic and austenitic.
The
austenitic stainless steels (300 series) were developed for
use in both mild and severe
corrosive conditions. Austenitic stainless
steels are used at temperatures that range from cryogenic
temperatures, where they exhibit high toughness, to elevated
temperatures, where they exhibit good oxidation resistance.
Because the austenitic materials are nonmagnetic, they are
sometimes used in applications where magnetic materials are
not acceptable.
Grade 316 has
excellent corrosion resistance in a wide range of media.
Better corrosion resistance than 302 and 304; resists sodium
and calcium brines; hypochlorite solutions, phosphoric acid;
and the sulfite liquors and sulfurous acids used in the paper
pulp industry.
Etching
Detail

Above
is a photo of the corner of a Silver Memory™ tribute.
The background (black) is a mirror polished surface. The
silver letters are the result of etching into that mirrored
surface.
Galvanic
Corrosion Table
Most
Corrodible
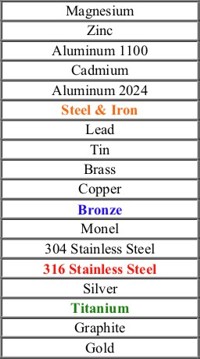
Least Corrodible
Galvanic
Compatibility
Often
when design requires that dissimilar
metals come in contact, the galvanic compatibility
is managed by finishes and plating. The finishing and plating
selected facilitate the dissimilar materials being in contact
and protect the base materials from corrosion.
For harsh
environments, such as outdoors, high
humidity, and salt environments fall into this category.
Typically there should be not more than 0.15 V difference
in the "Anodic Index". For example; gold
- silver would have a difference of 0.15V being acceptable.
For normal environments,
such as storage in warehouses or non-temperature and humidity controlled
environments. Typically there should not be more than 0.25 V difference
in the "Anodic Index".
For controlled environments,
such that are temperature and humidity controlled, 0.50 V can be
tolerated. Caution should be maintained when deciding for this
application as humidity and temperature do vary from regions.
Anodic
Index
|
Metallurgy
Index (V)
|
Gold,
solid and plated, Gold-platinum alloy
|
0.00
|
Rhodium
plated on silver-plated copper
|
0.05
|
Silver,
solid or plated; monel metal. High nickel-copper
alloys
|
0.15
|
Nickel,
solid or plated, titanium an s
alloys, Monel
|
0.30
|
Copper,
solid or plated; low brasses or bronzes; silver
solder; German silvery high copper-nickel alloys;
nickel-chromium alloys
|
0.35
|
Brass
and bronzes
|
0.40
|
High
brasses and bronzes
|
0.45
|
18%
chromium type corrosion-resistant steels (316L)
|
0.50
|
Chromium
plated; tin plated; 12% chromium type corrosion-resistant
steels
|
0.60
|
Tin-plate;
tin-lead solder
|
0.65
|
Lead,
solid or plated; high lead alloys
|
0.70
|
Aluminum,
wrought alloys of the 2000 Series
|
0.75
|
Iron,
wrought, gray or malleable, plain carbon and low
alloy steels
|
0.85
|
Aluminum,
wrought alloys other than 2000 Series aluminum,
cast alloys of the silicon type
|
0.90
|
Aluminum,
cast alloys other than silicon type, cadmium, plated
and chromate
|
0.95
|
Hot-dip-zinc
plate; galvanized steel
|
1.20
|
Zinc,
wrought; zinc-base die-casting alloys; zinc plated
|
1.25
|
Magnesium & magnesium-base
alloys, cast or wrought
|
1.75
|
Beryllium
|
1.85
|
You can see by the table above that
the 316L stainless would be compatible with brass/bronze,
even in a harsh environment.
The same can be said for titanium and brass/bronze.